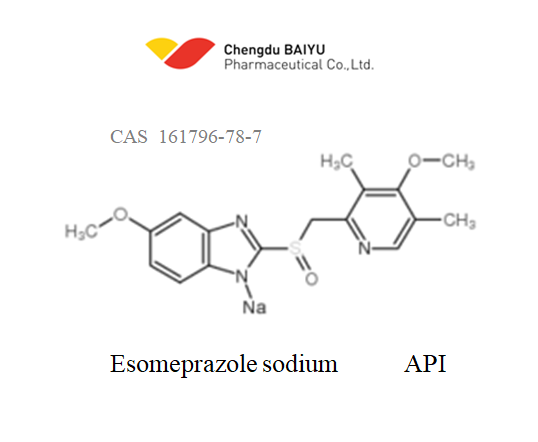
Category: API
Usage: It is used in the treatment of dyspepsia, peptic ulcer disease, gastroesophageal reflux disease, and Zollinger-Ellison syndrome.
Applications: Pharmaceutical raw materials
Place of Origin: China
Quality Specification: Please contact Baiyu for updated version COA

