2017年12月以来,国内多家公司相继提交CAR-T产品临床试验申请,3月1日,CDE更新一条受理消息,上海斯丹赛生物技术有限公司CAR-T产品ICT19G1临床试验申请获得CFDA承办受理,受理号CXSL1800020,这应该是国内第10家。笔者在这里分享一些关于斯丹赛CAR-T产品开发的主要进展。

一. 斯丹赛:从"高大上"到"接地气"
斯丹赛创始团队具有很强的学术背景,这个公司成立早期以TALEN相关技术及技术产品立足并开拓市场,得益于团队丰富的干细胞研发和基因编辑经验,公司早期客户主要是高校科研团队。
从"高大上"到"接地气",斯丹赛做了什么?团队在CAR-T产品开发上面更具有技术优势,就像公司官网讲到的,"人类胚胎干细胞和干细胞重编程技术是干细胞技术中最困难的,我们熟练掌握这两个技术后攻克CAR-T技术,类似于"军工"转"民用",驾轻就熟。"
斯丹赛目前公布哪些临床进展?
斯丹赛从"高大上"的干细胞研究转到CAR-T产品开发后,取得较好临床进展,部分数据公布如下:
1. 复发/难治性白血病41例多中心临床试验 34例完全缓解,33例MDR-
2. 3例人源CAR-T成人白血病治疗,完全缓解
3. 1例睾丸复发急淋白血病青年患者,完全缓解(Yu J, Hu Y, Pu C, et al. Successful chimeric Ag receptor modified T cell therapy for isolated testicular relapse after hematopoietic cell transplantation in an acute lymphoblastic leukemia patient[J]. Bone marrow transplantation, 2017, 52(7): 1065.)
目前斯丹赛的研发管线:
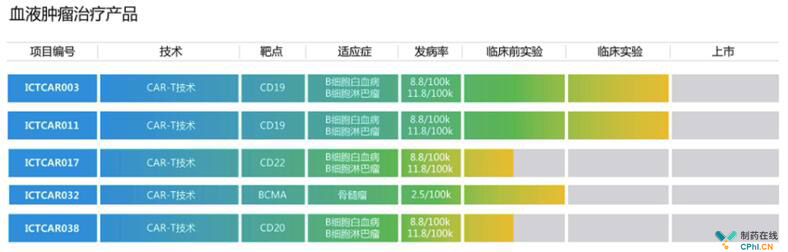
血液瘤:进展顺利 靶点CD19,CD20,CD22,BCMA
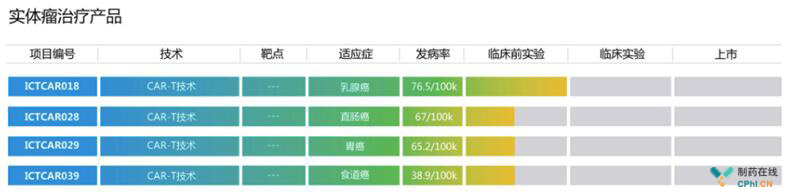
实体瘤:
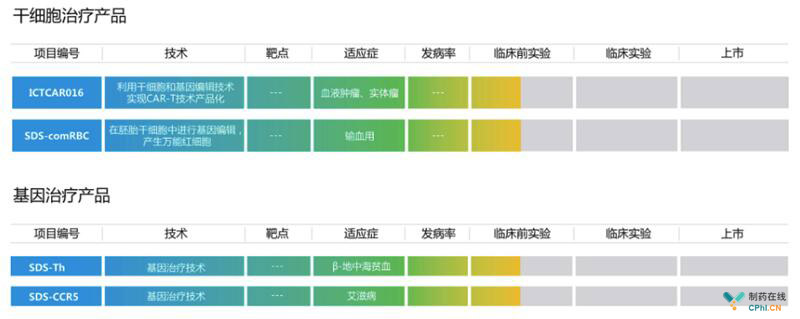
干细胞产品/基因治疗产品:
目前,斯丹赛CAR-T血液瘤产品无疑是开发最为顺利的,其主要合作单位是浙江大学医学院附属第一医院。
二. 斯丹赛专利分析
斯丹赛官网称已申请了26项欧美专利和5项PCT专利,笔者通过专利检索(可见附表),发现斯丹赛早期专利围绕基因编辑技术,比较重要的关于CAR-T产品的有《Humanized anti-CD19 antibody and use thereof 》《Activation and expansion of T cells 》《Reducing immune tolerance induced by PD-L1》等。斯丹赛对CAR-T产品做了改进,有几个点值得关注:
1. 减少PD-1/PD-L1所导致的免疫耐受 可见专利CN107073138 (A),专利对PD-1蛋白序列做了改造,从而减少PD-1/PD-L1所导致的免疫耐受;
2. 将抗CD19 抗体人源化 可见专利《Humanized anti-CD19 antibody and use thereof 》,这可能降低免疫原性,人源化CAR-T具有开发潜力。
3. CAR-T产品细胞内共刺激因子为4-1BB,4-1BB和CD28安全之争一直存在,但是数据看来,4-1BB在某些适应症中确实具有更好的安全性。
三.最后谈一谈ICT19G1
斯丹赛虽然还未透漏关于ICT19G1的相关信息,但是笔者根据斯丹赛公布临床数据及临床试验,推断ICT19G1应该是一款靶向CD19CAR-T细胞产品。
目前,clinical trials有两条斯丹赛信息,即NCT02813837(SCT019-01)和NCT03118180(CART19-001),可进入官网查看相关信息。
附表:斯丹赛专利一览
|
USE OF CHIMERIC ANTIGEN RECEPTOR MODIFIED CELLS TO TREAT CANCER |
Publication info: |
Priority date: |
|
|
US2017355776 (A1) |
01/04/2016 |
|
|
14/12/2017 |
|
|
Reducing immune tolerance induced by PD-L1 |
Publication info: |
Priority date: |
|
|
AU2016228080 (A1) |
02/03/2015 |
|
|
07/09/2017 |
|
|
|
KR20170124576 (A) |
|
|
|
10/11/2017 |
|
|
Method for directional cloning |
Publication info: |
Priority date: |
|
|
US2017218380 (A1) |
17/10/2014 |
|
|
03/08/2017 |
|
|
|
CN106795522 (A) |
|
|
|
31/05/2017 |
|
|
ACTIVATION AND EXPANSION OF T CELLS |
Publication info: |
Priority date: |
|
|
WO2017059796 (A1) |
08/10/2015 |
|
|
13/04/2017 |
|
|
HUMANIZED ANTI-CD19 ANTIBODY AND USE THEREOF |
Publication info: |
Priority date: |
|
|
WO2017015783 (A1) |
24/07/2015 |
|
|
02/02/2017 |
|
|
|
EP3149046 (A1) |
|
|
|
05/04/2017 |
|
|
|
EP3149046 (A4) |
|
|
|
30/08/2017 |
|
|
TRANSCRIPTION ACTIVATOR-LIKE EFFECTOR ASSEMBLY |
Publication info: |
Priority date: |
|
|
EP3118319 (A1) |
12/09/2012 |
|
|
18/01/2017 |
|
|
|
EP2864483 (A1) |
|
|
|
2015-04-29 |
|
|
|
EP2864483 (A4) |
|
|
|
2015-06-10 |
|
|
|
EP2864483 (B1) |
|
|
|
2017-02-15 |
|
|
|
EP2864483 (B8) |
|
|
|
26/04/2017 |
|
|
Modified cells for production of blood cells |
Publication info: |
Priority date: |
|
|
CN105518127 (A) |
06/09/2013 |
|
|
20/04/2016 |
|
|
Multi-module DNA (deoxyribonucleic acid) library and method for constructing transcription activator like effector nuclease plasmid |
Publication info: |
Priority date: |
|
|
CN103695452 (A) |
09/12/2013 |
|
|
2014-04-02 |
|
|
|
CN103695452 (B) |
|
|
|
11/01/2017 |
|
|
Single-module DNA (deoxyribonucleic acid) library and connecting method for TALENs (transcription activator-like effector nucleases) identification modules |
Publication info: |
Priority date: |
|
|
CN103497966 (A) |
27/09/2013 |
|
|
2014-01-08 |
|
|
|
CN103497966 (B) |
|
|
|
10/02/2016 |
|
|
A pair of transcription activator like effector nucleases of L3 and R1 and a coding gene and an application thereof |
Publication info: |
Priority date: |
|
|
CN102850444 (A) |
21/07/2012 |
|
|
2013-01-02 |
|
|
|
CN102850444 (B) |
|
|
|
18/02/2015 |
|
|
Recombinant transcription activator like effector, transcription activator like effector nuclease, as well as coding gene and application thereof |
Publication info: |
Priority date: |
|
|
CN102702335 (A) |
23/05/2012 |
|
|
2012-10-03 |
|
|
|
CN102702335 (B) |
|
|
|
09/07/2014 |
|
|
One pair of transcription activator effect factor nucleases R1 and R2, coding gene and application thereof |
Publication info: |
Priority date: |
|
|
CN102702332 (A) |
23/05/2012 |
|
|
2012-10-03 |
|
|
|
CN102702332 (B) |
|
|
|
08/01/2014 |
|
|
Pair of transcription activator-like effector nucleases (TALEN), encoding gene and application thereof |
Publication info: |
Priority date: |
|
|
CN102702331 (A) |
23/05/2012 |
|
|
2012-10-03 |
|
|
|
CN102702331 (B) |
|
|
|
06/08/2014 |
|
参考:
http://www.sidansai.com
作者简介:Dopine,注册执业药师,河南省药学会会员,就职于河南省某三级甲等医院PIVAS,精于临床药学服务,专注临床用药安全和不合理用药。对国内外医药审评审批政策,研发动态,新药注册审批等长期关注。



合作咨询
![]() 肖女士
肖女士
![]() 021-33392297
021-33392297
![]() Kelly.Xiao@imsinoexpo.com
Kelly.Xiao@imsinoexpo.com
 2006-2025 上海博华国际展览有限公司版权所有(保留一切权利)
沪ICP备05034851号-57
2006-2025 上海博华国际展览有限公司版权所有(保留一切权利)
沪ICP备05034851号-57