2019年09月10日,诺奖风向标之称的拉斯克奖揭晓,2019 拉斯克奖临床医学研究奖 (2019 Lasker-DeBakey Clinical Medical Research Award)授予了赫赛汀团队 (Axel Ullrich, Dennis Slamon, and Michael Shepard)。

2019 Lasker~DeBakey Clinical Medical Research Award
HER2是一个经典靶点,毫无疑问,罗氏深耕HER2多年,开发出曲妥珠单抗、帕妥珠单抗、恩美曲妥珠单抗重磅炸 弹级别药物,2018年3款产品销售额达到惊人的107.3亿瑞士法郎,药物给乳腺癌患者带来巨大的临床获益!
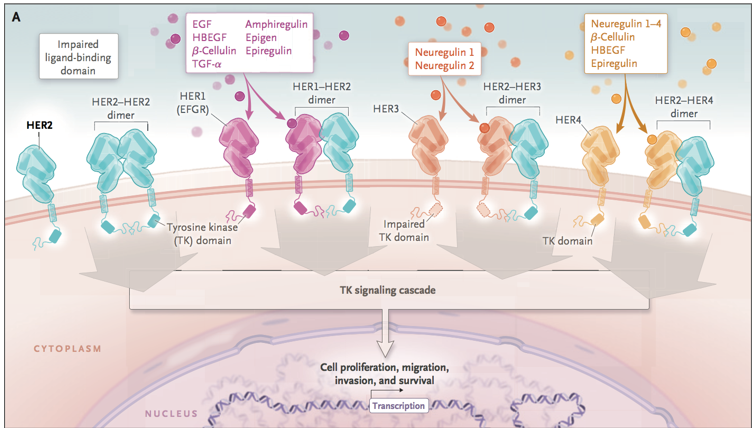
HER/EGFR/ERBB family [1]
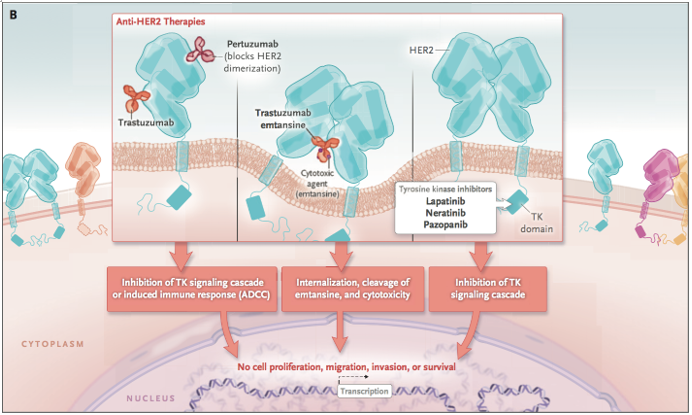
曲妥珠单抗、帕妥珠单抗、恩美曲妥珠单抗作用机制[1]
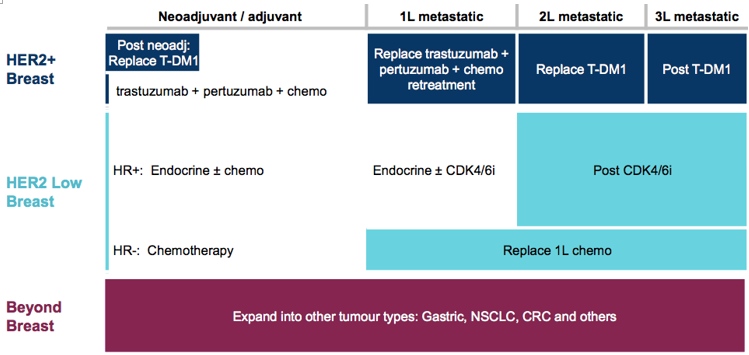
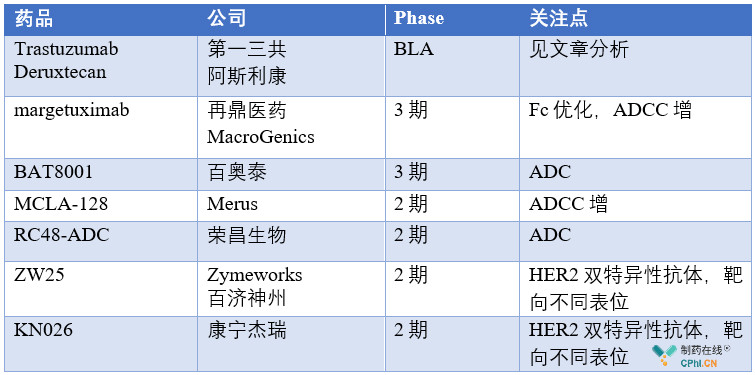
该领域内除了罗氏的多款单抗和抗体偶联药物外,新一代靶向HER2大分子药物的开发从未停歇,同时仍有着极大的未满足的临床需求,目前值得关注的产品包括Trastuzumab Deruxtecan,margetuximab,BAT8001,ZW25,KN026,RC48-ADC等。新一代的HER2大分子药物中,ADC、双抗产品涌现出多个潜力品种,这其中,最为令人关注的便是刚刚递交上市申请的第一三共/阿斯利康HER2-ADC药物Trastuzumab Deruxtecan!借此机会,文章重点介绍了Trastuzumab Deruxtecan,并简单梳理了新一代靶向HER2大分子药物中的潜力药物。
一.Trastuzumab Deruxtecan给HER2+肿瘤带来突破
2019年09月09日,第一三共和阿斯利康联合开发的HER2-ADC药物Trastuzumab Deruxtecan (DS-8201) 率先在日本递交上市申请,适应症Kadcyla经治的HER2+转移性乳腺癌,注册临床试验DESTINY-Breast01。这是该药物一个里程碑进展,阿斯利康69亿美元重金购入的肿瘤资产正式开始商业化。
Trastuzumab Deruxtecan (DS-8201)
Trastuzumab Deruxtecan (DS-8201) 是一款靶向HER2的抗体偶联药物,相比Kadcyla (首个获批的HER2-ADC),DS-8201创新了偶联的小分子,具有更强的穿透性、更高的DAR。
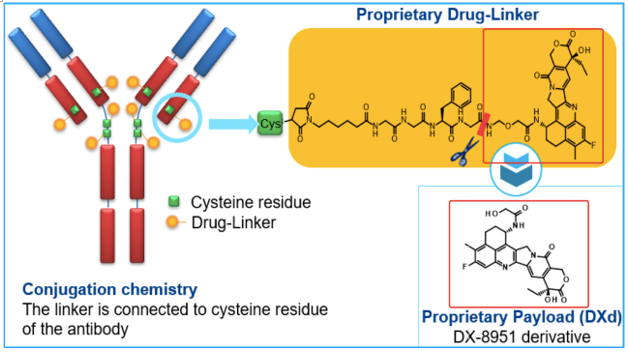
DS-8201 阿斯利康官网
Trastuzumab Deruxtecan (DS-8201):给经治的HER2+乳腺癌患者带来明显临床获益
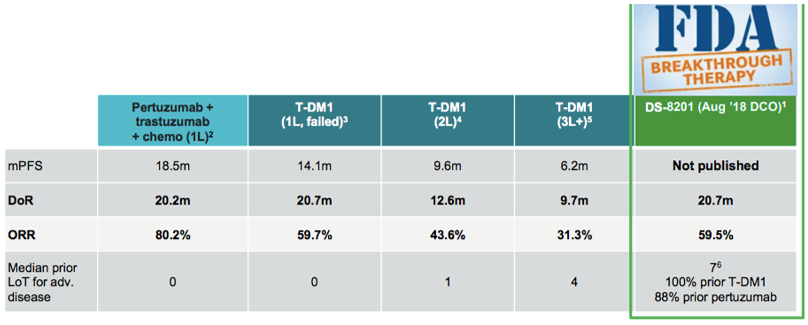
阿斯利康和第一三共预计2019年公布注册临床试验DESTINY-Breast01关键临床数据,这是需要关注的一个点。
二、DS-8201的市场潜力巨大
DS-8201具有广阔的市场前景,具有革新目前标准疗法的强大实力。
2019年,DS-8201预计向FDA,NMPA递交上市申请,另外,预计将会公布DESTINY-Breast01,DESTINY-Gastric01关键数据。
三.HER2靶点仍值得继续深耕
阿斯利康官网
HER2是经典靶点,市场上有着曲妥珠单抗、帕妥珠单抗、T-DM1等多款单抗和抗体偶联药物,但是仍有着巨大的仍未满足的临床需求,除了已上市产品外,临床开发中的多款产品值得特别关注,开发中的药物均有着潜在的更优临床疗法,尤其是Trastuzumab Deruxtecan。
作者简介:1°C,医药行业从业人员,希望自己的专业文字会越来越有温度,医药知识能够服务更多人,打破信息知识的壁垒!
1 HER2 and Breast Cancer - A Phenomenal Success Story
附:2019 Lasker-DeBakey Clinical Medical Research Award
Herceptin-a targeted antibody therapy for breast cancer
For their invention of Herceptin, the first monoclonal antibody that blocks a cancer-causing protein, and for its development as a life-saving therapy for women with breast cancer
The 2019 Lasker~DeBakey Clinical Medical Research Award honors three scientists—H. Michael Shepard, Dennis J. Slamon, and Axel Ullrich —who invented Herceptin, the first monoclonal antibody that blocks a cancer-causing protein, and developed it into a life-saving therapy for women with breast cancer. The innovation reduces the risk of recurrence and extends survival time for patients with metastatic as well as early-stage disease. Every year, more than 50,000 women in the US are diagnosed with the type of breast cancer that the drug attacks, and over 2.3 million individuals have received the treatment since it became available. Shepard (now at BetterOutcomes4Cancer) and Ullrich (now at Max Planck Institute of Biochemistry, Martinsried, Germany) conducted their Herceptin investigations at Genentech. Slamon did his at the University of California, Los Angeles (UCLA), where he continues to work.
In the mid 1970s, scientists discovered that genes within our own bodies can trigger cancer. This revelation sparked the idea that stifling the activities of such oncogenes might thwart the trouble they instigate. This approach—in which a therapy would fixate on molecules that dwell specifically in cancer cells and also drive malignancy—held great appeal. Such a targeted strategy might avoid many of the harsh side effects associated with chemotherapy while striking the source of the affliction.
They saw HER standing there
Prominent among known oncogenes were several that encode overzealous versions of growth factors and the receptors that sense them. The receptors, which lie across the cell membrane, receive signals from the external environment and then relay them inside to spur proliferation. Ullrich was particularly interested in a subgroup of these proteins—the tyrosine kinase receptors—and he wanted to uncover previously unknown members of this molecular family in humans.
In 1985, Ullrich, Arthur Levinson (also at Genentech), and colleagues found a gene whose sequence resembles that of the human epidermal growth factor receptor (EGFR or HER1) and the chicken oncogene v-erbB. They named it HER2—human EGF receptor 2—because of this similarity. Its sequence and location on the chromosome suggested that it might be the human version of neu, a rat oncogene discovered the year before by Robert Weinberg (Massachusetts Institute of Technology). Independently and simultaneously, two other groups, led by Stuart Aaronson (National Cancer Institute) and Tadashi Yamamoto (University of Tokyo), identified HER2 and showed that it was amplified in malignancies from a human breast and a salivary gland.
In the meantime, UCLA oncologist Slamon and, independently, the late William McGuire (University of Texas Medical Center, San Antonio), had been collecting cancer tissues that had been surgically removed for therapeutic reasons and checking whether established oncogenes were firing excessively in them. Ullrich, with his tantalizing gene, and Slamon, with his human tumor library, connected. In 1987, they reported that almost 30% of 189 breast cancer samples contained more than one copy of the HER2 gene. Analysis of health records for many of the patients represented in the study revealed that women whose tumors carried multiple copies of the gene relapsed more quickly and died sooner than did those whose tumors contained only one copy. Furthermore, the gene amplification better predicted prognosis than did most standard indicators, such as tumor size and hormone receptor status.
These associations were provocative, but numerous previously described proteins appeared on cancer cells yet did not propel tumor formation or progression. To gain candidacy as a therapeutic target, HER2 overabundance would have to cause, not just correlate with, malignancy.
To investigate this issue, Ullrich and postdoctoral fellow Robert Hudziak engineered mouse connective-tissue cells grown in laboratory culture dishes to produce extra HER2 protein. The supplemental HER2 caused the cells to keep dividing under conditions in which they’d usually stop, a sign of transformation to a cancerous state. The HER2-augmented cells also displayed other evidence of malignancy, and they formed tumors after injection into mice. Because the only difference between the corrupt and well-mannered cells was the amount of HER2, these results indicated that surplus growth factor receptor unbridles normal restraints on replication.
Simultaneously and in parallel, Shepard, in his own lab at Genentech, had landed on HER2 from a different direction. He was probing the mechanisms by which cancer cells evade destruction by a chemical called Tumor Necrosis Factor-α (TNF-α), a component of the body’s immune response. He had found that activation of some tyrosine kinases can curb TNF-α’s lethal potency. Together, he and Ullrich showed that HER2 renders mouse cells resistant to TNF-α’s toxic effects. Perhaps, they reasoned, HER2 promotes aggressive cancers not only by causing growth to run amok, but also by toughening up the cells and endowing them with ways to withstand assaults that normally would kill them.
If excessive quantities of HER2 cause runaway growth and protection from the body’s defense system, quashing receptor function might reverse these aberrant behaviors. The part of the protein that resides outside the cell was especially alluring because it was likely to be accessible to a drug. To block HER2 stimulation, the researchers wanted to develop a compound that would bind to this exposed portion and impede incoming signals. They sought monoclonal antibodies to serve that purpose.
HER promise revealed
The Genentech scientists made a collection of monoclonal antibodies in mice and zeroed in on one that inhibited growth of several breast cancer cell lines. Crucially, it had this effect only on cell lines that overproduce HER2 protein. Furthermore, it sensitized these tumor cells to killing by TNF-α. The top pick antibody, dubbed 4D5, did not bind to HER2’s relative, the human EGF receptor—an important characteristic, as a successful drug must not interfere with activities of healthy cells. These observations suggested that a 4D5-based treatment would foil only HER2-overexpressing cells.
Even if 4D5 could, in principle, crush tumor growth in people’s bodies, a significant hurdle remained. Because it came from mice, the human immune system would deem it “foreign” and destroy it. To design an antibody that would not stir such an inflammatory reaction, Paul Carter, working with Shepard, aimed to retain the bare minimum of 4D5—only the part that binds HER2—and replace the rest with human antibody sequences. In 1992, the team reported that one of these “humanized” antibodies bound HER2 three times more tightly than 4D5 and blocked proliferation of a HER2-overexpressing breast cancer cell line as efficiently.
Despite these auspicious results, hesitation lurked among Genentech’s decision makers. The company had pursued other potential cancer drugs that had not panned out. Furthermore, many experts were skeptical that monoclonal antibodies would prove therapeutically useful against cancer, especially for solid rather than blood malignancies—in part because scientists doubted that these agents would penetrate the tumors. To bolster the case for clinical pursuit, Shepard and Slamon performed numerous studies. They showed, for instance, that radioactive-tagged mouse antibody—a single dose, so it would not elicit a strong immune response—accumulated in tumors, but only in those of women with cancers that overproduced HER2. This encouraging observation energized the team, as did the patients’ willingness to participate even though they would not benefit and might suffer unknown consequences. Such generosity repeated itself at numerous steps of the enterprise.
During this period, the investigators also collected data that would help guide clinical deployment of the potential drug. Using human breast cancer cells grown in the lab, Slamon and colleagues explored how best to combine the antibody with standard chemotherapy regimens. Genentech scientists and Slamon also developed diagnostic tests to identify women with HER2-overexpressing (HER2+) breast cancers.
Troublemaker transformed into target
The company decided to move forward, and clinical trials began on women with metastatic HER2+ breast cancer. By 1998, the data were strong enough that the humanized monoclonal antibody—now called trastuzumab (brand name, Herceptin)—gained approval by the U.S. Food and Drug Administration (FDA).
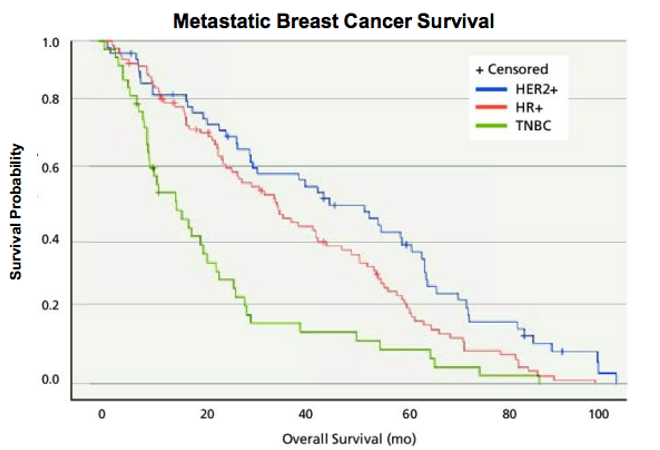
In 2001, Slamon and colleagues published those results. A large, randomized study on women with progressive, HER2+ breast cancer revealed that the addition of Herceptin to chemotherapy stalled disease progression, reduced the one-year death rate from 33 percent to 22 percent, and extended overall survival. Subsequent trials on early-stage, HER2+ breast cancer showed that Herceptin also improves outcomes in that setting, for which it was FDA-approved in 2006 (see Figure).
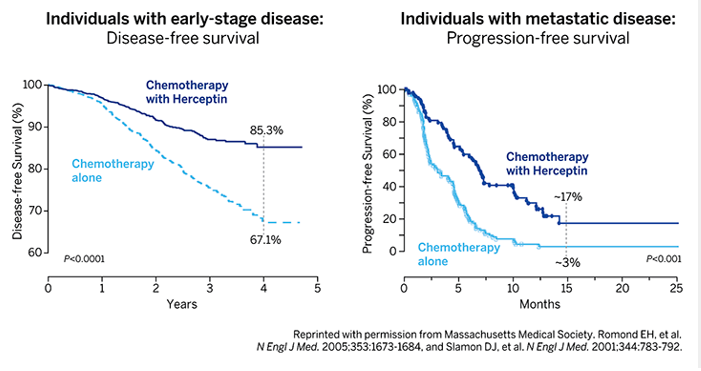
Beating back breast cancer
Women with early-stage (left) or metastatic (right) HER2+ breast cancer were treated with either Herceptin plus chemotherapy or chemotherapy alone. The percentage of women with early-stage breast cancer who were alive and free of disease four years after beginning the trial was significantly higher if they received Herceptin (85.3 %) than if they did not (67.1 %). The percentage of women whose metastatic disease had not progressed 15 months after beginning the trial was significantly higher if they received Herceptin (~17.5%) than if they did not (~3%).
Herceptin is now a standard therapy for HER2+ breast cancers. Researchers have continued to investigate HER2, and additional antibodies that target it have reached the market. For instance, a drug called pertuzumab differs from Herceptin in the region of HER2 that it binds and its mechanism of action. A 2015 review reported that overall survival of women with metastatic HER2+ disease who received chemotherapy plus pertuzumab and Herceptin was more than 4.5 years. In 2001, life expectancy for women with that diagnosis was 1.5 years.
The impact of Herceptin’s development reaches beyond breast cancer by establishing that monoclonal antibodies can indeed combat solid tumors. Furthermore, the innovation offers a new model for personalized medicine by deploying a diagnostic test that identifies the most appropriate patients to treat with a particular intervention. By uncovering and exploiting the molecular pathology of a devastating disease, Shepard, Slamon, and Ullrich conceived and executed a new blueprint for drug discovery that has already bestowed tens of thousands of women with time and quality of life.
by Evelyn Strauss
Key Publications on Herceptin – Its Invention and Clinical Development
Coussens, L., Yang-Feng, T.L., Liao, Y.-C., Chen, E., Gray, A., McGrath, J., Seeburg, P.H., Libermann, T.A., Schlessinger J., Francke, U., Levinson, A., and Ullrich, A. (1985). Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science. 230, 1132-1139.
Hudziak, R.M., Schlessinger, J., and Ullrich, A. (1987). Increased expression of the putative growth factor receptor p185-HER2 causes transformation and tumorigenesis in NIH 3T3 cells. Proc. Natl. Acad. Sci. 84, 7159-7163.
Slamon, D.J., Clark, G.M., Wong, S.G., Levin, W.J., Ullrich, A., and McGuire, W.L. (1987). Human breast cancer: correlation of relapse and survival with amplification of the HER2 protooncogene. Science. 235, 177-182.
Hudziak, R.M., Lewis, G.D., Shalaby, M.R., Eessalu, T.E., Aggarwal, B.B., Ullrich, A., and Shepard, H.M. (1988). Amplified expression of the HER2/ERBB2 oncogene induces resistance to tumor necrosis factor-alpha in NIH 3T3 cells. Proc. Natl. Acad. Sci. USA. 85, 5102-5106.
Slamon, D.J., Godolphin, W., Jones, L.A., Holt, J.A., Wong, S.G., Keith, D.E., Levin, W.J., Stuart, S.G., Udove, J., Ullrich, A., and Press, M.F. (1989). Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science, 244, 707-712.
Hudziak, R.M., Lewis, G.D., Winget, M., Fendly, B.M., Shepard, H.M., and Ullrich, A. (1989). p185HER2Monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol. Cell. Biol. 9, 1165-1172.
Shepard, H.M., Lewis, G.D., Sarup, J.C., Fendly, B.M., Maneval, D., Mordenti, J., Figari, I., Kotts, C.F., Palladino, Jr., M.A., Ullrich, A., and Slamon, D. (1991). Monoclonal antibody therapy of human cancer: taking the HER2 protooncogene to the clinic. J. Clin. Immunol. 11, 117-127.
Carter, P., Presta, L., Gorman, C.M., Ridgeway, J.B., Henner, D., Wong, W.L., Rowland, A.M., Kotts, C., Carver, M.E., and Shepard, H.M. (1992). Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc. Natl. Acad. Sci. USA. 89, 4285-4289.
Pegram, M.D., Lipton, A., Hayes, D.F., Weber, B.L., Baselga, J.M., Tripathy, D., Baly, D., Baughman, S.A., Twaddell, T., Glaspy, J.A., and Slamon, D.J. (1998). Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185 HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J. Clin. Oncol. 16, 2659-2671.
Slamon, D.J., Leyland-Jones, B., Shak, S., Fuchs, H., Paton, V., Bajamonde, A., Fleming, T., Eiermann, W., Woldter, J., Pegram, M., Baselga, J., and Norton, L. (2001). Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 344, 783-792.
Shepard, H.M., Jin, P., Slamon, D.J., Pirot, Z., and Maneval, D.C. (2008). Herceptin. In Therapeutic Antibodies. Handbook of Experimental Pharmacology. Edited by Y. Chernajovsky and A. Nissim, Springer-Verlag, Berlin, Heidelberg. pp. 183-219.



合作咨询
![]() 肖女士
肖女士
![]() 021-33392297
021-33392297
![]() Kelly.Xiao@imsinoexpo.com
Kelly.Xiao@imsinoexpo.com
 2006-2025 上海博华国际展览有限公司版权所有(保留一切权利)
沪ICP备05034851号-57
2006-2025 上海博华国际展览有限公司版权所有(保留一切权利)
沪ICP备05034851号-57