雪随流年花开尽,茗香自然迎春风。虽然撰写此文时,中国的北方还是白雪皑皑的样子;但是作者坚信,目前的困难和疑惑是暂时的,终将在春天来临时会逐渐消融。
关于中国企业如何应对国家药品标准变更带来的挑战,作者在《兔年谈之十五-药品标准管理办法的尴尬》一文中论述了目前国内关于药品标准变更管理政策的模糊不清。这个问题具有全球性质,其他国家或者地区的制药企业,也会遇到类似问题。本文就介绍一下欧盟制药企业遇到类似问题的应对方案。
第一部分:EMA关于欧洲药典实施的问答
2023年10月30日,EMA发布《Q & A on implementation of Ph.Eur. Medicinal Product Monographs》。
1.Should a finished product for which a Ph.Eur. Medicinal Product Monograph (MPM) is official comply with the requirements of that Monograph
如果欧洲药典(缩写Ph.Eur.)收载了制剂的各论(MPM),是否这个制剂药品应该符合该各论的要求?
In principle, finished products for which a Ph.Eur. Medicinal Product Monograph (MPM) is official should comply with the requirements described in the Monograph, unless otherwise prescribed in General Notices of the Ph.Eur.
原则上,如果一种制剂药品存在欧洲药典的制剂各论(MPM)的官方版本,那么该制剂药品应符合各论中描述的要求,除非在欧洲药典的凡例中另有规定。
Different scenarios exist:
存在如下的各类特殊情形:
In case the MPM is official before the medicinal product is approved, the finished product has to comply with the requirements described in the Monograph. In this case the applicants have possibility and time to take the MPM specifications and analytical methods into account when setting specifications and developing suitable analytical methods for the specific medicinal product.
如果药品制剂获批前,药品制剂各论(MPM)已经获得官方认可,成品必须符合所述各论中描述的要求。在这种情况下,申请人在为特定药品设置标准和开发适当的分析方法时,有可能性和工作时间考虑到MPM中收载的标准和分析方法。
In case the medicinal product is approved before the MPM is developed, the MPM should have been developed to embrace the quality of all approved medicinal products. If this has not been the case and the finished product specification is wider than the range described in the MPM, this should be communicated to the NPA (National Pharmacopoeia Authority) and EDQM (European Directorate for the Quality of Medicines & HealthCare) to be considered for inclusion in a revised monograph.
如果药品在药品制剂药典各论制定之前获得批准,药品制剂药典各论应该被制定,以涵盖所有已批准药品的质量。如果这种情况没有发生,并且成品的标准范围超出了药品药典各论中描述的范围,应该向国家药典委员会和EDQM报告,以便考虑将其纳入修订的药典。
See also questions below for more details.
参见下面问题以获得更多细节。
2. How can the compliance with the Ph.Eur. monograph for a medicinal product be demonstrated during a MA procedure, when alternative methods are proposed in the MA dossier
如何在MA申报资料中提出了替代方法时,在MA(上市申请)流程中证明药品符合欧洲药典各论对药品的规定?
In order to demonstrate compliance with the Ph. Eur. MPM, the applied finished product should be tested against the Ph. Eur. MPM using same analytical methods (except for dissolution, see Question 3 below).
为了证明符合欧洲药典关于药品各论的要求,应该使用相同的分析方法对所使用的成品进行测试(除了溶出度测试,详见第3个问题)。
It should be demonstrated whether the Ph. Eur. analytical methods are suitable for controlling the specific product in question (composition, manufacturing method and container closure system etc.). It should be demonstrated (preferably in CTD Module 3.2.P.5), whether the MPM actually controls the entire impurity profile of the finished product (i.e. all relevant impurities, degradation products, nitrosamines) or not.
需要证明Ph. Eur. 的分析方法是否适用于特定产品的质控要求(包括组分、制造方法和容器密封系统等)。最好在CTD模块3.2.P.5中进行证明,MPM是否实际控制了成品的整个杂质谱(例如所有相关杂质、降解产物和亚硝胺)。
According to the General Notices of the Ph. Eur., alternative methods (i.e: in-house methods) may be used for control purposes, provided that the methods used enable an unequivocal decision to be made as to whether compliance with the standards of the monographs would be achieved if the official methods were used. The methods of analysis confirm compliance with corresponding Ph.Eur. criteria, if tested. (see also Question 4 and 5).
根据《欧洲药典》的凡例规定,可以使用替代方法(即内部方法)进行质控目的,前提是所使用的方法能够明确地判断是否符合使用官方方法所可达到的药典标准。如果采用替代分析方法测试,需要能够证实符合相应的《欧洲药典》标准。(参见问题4和问题5)
“It is acknowledged that additional controls may be required to monitor degradation products other than those controlled by the monograph (e.g. degradation products related to different excipients or containers used, or from a different manufacturing process).” (Ph. Eur. General Notices 1.5.3.1.)
需要认识到除了药典所控制的降解产物项目之外,可能需要额外的控制措施来监测与不同辅料、容器或制造工艺相关的降解产物。(Ph. Eur.凡例1.5.3.1.)
3. Is it necessary to demonstrate the compliance with the dissolution test and requirements recommended in the Ph.Eur. MPM
是否需要证明符合欧洲药典中收载的药典各论中建议的溶出度测试和要求?
Test and requirements for dissolution in MPMs are minimum requirements and not mandatory per se (see Ph. Eur. General Notices). For dissolution test, the specification should be derived from the dissolution results of the bio batch(es) in the individual marketing authorization procedure and the dissolution method should be discriminatory for the specific product (manufacturing process/product formulation).
药典各论(MPMs)中的溶出度测试和要求是最低要求,不是强制要求(详见欧洲药典的凡例)。对于溶出度测试,标准应该基于个别上市许可程序中生物研究批次中的溶出结果,并且溶出方法应该对特定产品(生产工艺/产品配方)具有区分性。
Broadening the Q-value can only be justified by in-vivo data. It is important to note that the dissolution test described in the respective Ph. Eur. Medicinal Product Monographs can only be used if the applicant has demonstrated the suitability (including discriminatory power) of the test for the given product to the satisfaction of the competent authority. In any case, the dissolution test proposed should be approved by the competent authority in the context of the marketing authorization procedure.
将Q值放宽范围只能通过体内数据证明合理性。需注意,在欧洲药典药品各自各论中描述的溶解度测试只有在申请人向主管机构证明该测试适用于给定产品(包括区分能力)后才能使用。无论如何,所提议的溶解度测试都需在上市许可程序的背景下获得主管机构的批准。
Demonstration of compliance with the monograph dissolution test is not required as part of a MAA or MAV. However, when tested, the medicinal product has to comply with the monograph dissolution test, unless otherwise justified by the applicant.
证明符合药典各论的溶出度测试不是上市申请(MAA)或上市许可变更(MAV)的一部分要求。然而,一旦进行了测试,除非申请人提出了合理的理由,否则药品必须符合药典各论的溶出度测试的要求。
Further guidance on the dissolution test can be found in the Ph. Eur. General Notices (1.5.3.2).
有关溶出度试验的进一步指导可以在欧洲药典凡例(1.5.3.2)中找到。
4. Should the already approved in-house specification be changed to the one described in the MPM
是否应该将已经批准的内部标准变更为MPM中描述的标准吗?
For medicinal products already on the market with a valid marketing authorization licence, in case the approved shelf life specification limits are in line with or stricter than the Medicinal Product monograph there is no need to change the specification(s) limits and methods.
对于已经在市场上获得有效上市许可证的药品,如果批准的有效期标准限度与药品专论要求一致或更为严格,就无需改变标准限度和方法。
Alternative methods (i.e: the already approved in-house methods) may be used for control purposes, provided that the methods used enable an unequivocal decision to be made as to whether compliance with the standards of the monographs would be achieved if the official methods were used. The methods of analysis confirm compliance with corresponding Ph.Eur. criteria, if-tested.
可以使用替代方法(即:已经批准的内部方法)来进行控制,前提是使用的方法能够明确地判断是否能够达到采用官方方法实现的药典各论标准的要求。如果使用替代方法,要证明符合相应的欧洲药典标准。
If higher/wider limits than indicated in the Medicinal Product Monograph (limits compared using the same analytical method) have been approved for a product, and these are justified in line with current ICH/EU requirements and actual batch data, these should be communicated to the NPA and EDQM to be considered for inclusion in a revised monograph (see also Question 6 below).
如果产品的批准限度高于或宽于药品药典各论中所示的限度(使用相同的分析方法进行比较),并且满足当前ICH/EU的要求和实际批次数据的要求,应将其通知给国家药典委员会和EDQM,以便考虑将其纳入修订后的药典各论(详见下面的问题6)。
However, in case the higher/wider limits of the product are not fully justified by batch data, tightening the limits to the MPM’s requirements is needed by submission of a variation application.
然而,如果产品的较高/较宽限度不能通过批次数据完全证明合理性,就需要通过提交变更申请将限度调整到药品药典各论(MPM)的要求水平。
‘It should be noted that the specification limit is specific for the respective analytical method and therefore, limits cannot be compared across different analytical methods’.
应该注意的是,标准限度是特定于各自的分析方法的,因此不能在不同的分析方法之间进行比较。
5. Should the in-house developed methods be changed to the methods described in the MPM
公司内部开发的方法是否应该变更为MPM中描述的方法?
No. The product should comply with the requirements of the Medicinal Product Monograph (MPM) see Question 1, but it does not mean that it is mandatory for the manufacturer to use the monograph methods.
不。产品应符合药品药典各论的要求(参见问题1),但这并不意味着制造商必须使用药典各论的方法。
According to the Ph. Eur. General Notices, alternative methods (i.e: in-house methods) may be used for control purposes, provided that the methods used enable an unequivocal decision to be made as to whether compliance with the standards of the monographs would be achieved if the official methods were used. The methods of analysis confirm compliance with corresponding Ph.Eur. criteria, if-tested.
根据欧洲药典的凡例,可以使用替代方法(即:内部方法)进行质控目的,前提是所使用的方法能够明确地判断是否可以达到当使用官方方法时所实现的各论标准的合规要求。如果采用替代方法,应该证明分析方法在测试时确认符合相应的欧洲药典标准。
According to the Ph. Eur. General Notice, alternative methods can be used:
根据欧洲药典凡例,替代方法可以使用:
“1.1.2.5. Alternative methods. The tests and assays described are the official methods upon which the standards of the Pharmacopoeia are based. With the agreement of the competent authority, alternative methods of analysis may be used for control purposes, provided that the methods used enable an unequivocal decision to be made as to whether compliance with the standards of the monographs would be achieved if the official methods were used. In the event of doubt or dispute, the methods of analysis of the Pharmacopoeia are alone authoritative.” (General Notices of Ph.Eur.)
欧洲药典凡例1.1.2.5.替代方法:
所描述的测试和分析方法是药典标准所依据的官方方法。在有关部门的同意下,可以使用替代的分析方法进行质量控制,前提是这些方法能够明确判断如果使用官方方法是否符合药典标准。在存在疑问或争议的情况下,药典的分析方法是唯一的权威。
For dissolution testing see Question 3 above.
对于溶出度测试,参见上面的问题3.
6. Is it possible to authorize a finished product with wider limits than those laid down in the MPM
当一个药品的限度比药典各论上的限度更宽,是否可以被批准?
A medicinal product with wider limits than those laid down in the monograph could be authorized only in very exceptional cases.
只有非常少见的情况下,当一个药品标准限度比药典各论限度更宽,才可以被批准。
In case the proposed wider limits than those laid down in the monograph (limits compared using the same analytical method) are appropriately justified by the applicant in line with current ICH/EU requirements (by respective preclinical/toxicological studies/references, proper development, excipients, packaging material, actual batch analysis and stability data etc.) the marketing authorisation could be granted.
如果申请人能够按照当前ICH/EU的要求,通过相应的临床前/毒理学研究/参考资料、适当的开发、辅料、包装材料、实际批次分析和稳定性数据等充分证明所建议的限度范围比药典规定的范围更宽(使用相同的分析方法进行比较),则可能获得上市许可。
According to the General Monograph on Pharmaceutical Preparations (2619) section “Related substances”: “In exceptional circumstances and if justified by the applicant to the satisfaction of the competent authority, the latter may approve a wider limit than that described in the monograph. In these rare cases, the competent authority shall bring this to the attention of the Ph. Eur. Commission for review of the monograph and, where appropriate, its revision”.
根据欧洲药典《药用制剂通则》(2619)中“有关物质”部分的规定:“在特殊情况下,如果申请人能够向主管机关证明,并获得其认可,后者可以批准比药典各论中所述的更宽松的限度。在这些罕见情况下,主管机关应将此事提交给欧洲药典委员会审查各论,并在必要时进行修订。”
7. Is it necessary to submit variation application in order to demonstrate compliance with the MPM
为了证明符合欧洲药典各论的要求,是否有必要提交变更申请?
If no change is required, a variation application does not need to be submitted, but the supportive data (including suitability of the MPM methods) demonstrating compliance of the existing product with the MPM should be available at the manufacturing/control site. It should be kept in mind that the product should comply with the requirements of the MPM if tested (e.g. by an OMCL).
如果不需要进行任何变更,就无需提交变更申请,但应该在生产/质控现场准备好支持性数据(包括符合MPM适用性评估的方法),以证明现有产品符合MPM的要求。需要注意的是,如果进行测试(例如由OMCL进行测试),产品应符合MPM的要求。
If any changes are needed, they should be submitted in line with the EC variation classification guideline.
如果存在任何变更,应该根据EC变更分类指南提交变更。
8. Which type of variation should be used for changing the specification and/or analytical methods to those described in the MPM
应采用哪种类型的变更来将标准和/或分析方法变更为欧洲药典各论(MPM)中描述的那些方法?
The classification of the variation should correspond to the existing sub-categories of the EC Variation Classification Guideline in sections:
药品变更的分类应与欧洲委员会(EC)变更分类指南中已有的子类别相对应。
B.II.d.1 “Change in the specification parameters and/or limits of the finished product” or B.II.d.2 “Change in test procedure for the finished product”
B.II.d.1变更制剂产品的标准参数和/或限度;
B.II.d.2变更制剂产品的分析规程;
According to CMDh Q&A (List for the submission of variations according to Commission Regulation (EC) 1234/2008) point 3.8.:
根据CMDh问答文件《List for the submission of variations according to Commission Regulation (EC) 1234/2008》的3.8点:
Variation B.III.2 only relates to active substances, excipients, immediate packaging materials and active substance starting materials. Changes to comply with Ph.Eur. or with a national pharmacopoeia of a Member States affecting the finished product should be submitted according to the relevant variations listed under B.II. d.
变更分类B.III.2仅涉及活性物质、辅料、内包装物料和活性物质起始物料。为了符合欧洲药典或成员国国家药典的要求,并影响成品制剂的变更应根据B.II. d下列出的相关变更进行提交。
9. Is it necessary to submit variation application if the approved shelf life limits are in line with or stricter than the MPM
如果批准的有效期限度符合或者更严于MPM的要求,是否有必要提交变更申请?
No. In order to avoid the numerous unnecessary variation applications, it is not recommended to change the already approved specifications and/or the method in case the approved shelf-life limits are in line with or stricter than the MPM. The approved specification has already been evaluated and authorized according to EU/ICH guidelines and the obtained batch/stability data using validated analytical methods.
不。为了避免大量不必要的变更申请,在已批准的质量标准和/或方法与药典各论要求(MPM)相符或更严格的情况下,不建议进行更改。已批准的质量标准已根据欧盟/ICH指南和使用经过验证的分析方法获得的批次/稳定性数据进行评估和授权,不需要再次评估。
10. Is it possible to widen the specification limits to be the same as specified in the MPM
是否有必要为了和MPM要求保持一致而放宽标准限度?
Impurities/related substances: For already authorized products compliance with a monograph does not mean that the limits for impurities/related substances have to be widened. The specification represents process capability/product performance associated with safety and efficacy. Therefore, wider limits than previously approved need sound justification.
杂质/有关物质:对于已经获得批准的产品,遵守一项药典并不意味着杂质/有关物质的限度必须扩大。标准代表着与安全性和功效相关的工艺能力/产品性能。因此,放宽以前批准的限度需要有充分的正当理由。
Dissolution: No. For dissolution test, the specification should be derived from the dissolution results of the biobatch(es) in the individual marketing authorization procedure and the dissolution method should be discriminatory for the specific product (manufacturing process/product formulation).
溶出度:不需要。对于溶出度测试,标准应该根据个别上市许可程序中生物批次的溶出结果来确定,并且溶出方法应该对特定产品(制造工艺/产品配方)具有区分性。
Broadening the Q-value can only be justified by in-vivo data.
It is important to note that the dissolution test described in the Ph. Eur. MPM can only be used if the applicant has demonstrated the suitability of the test for the given product to the satisfaction of the competent authority. In any case, the dissolution test proposed should be approved by the competent authority in the context of the MAA assessment.
放宽“Q值”只能通过体内数据来证明合理性。需注意,欧洲药典中所描述的溶出度测试方法只有在申请人向主管机构证明该测试对特定产品的适用性并令其满意的情况下才可使用。无论如何,在MAA申请评估的背景下,所提出的溶出度测试方法都需获得主管机构的批准。
11. Which variation category should be used to widen the specification limits to be the same as specified in the MPM
为了放宽标准限度和MPM要求保持一致,需要采用什么变更分类?
Change (widening) the impurity or assay limits of the finished product to the limits in the Ph. Eur. monograph: Type II, B.II.d.1.e (Change outside the approved specifications limits range).
变更(放宽)制剂的杂质或者含量限度和欧洲药典各论保持一致,需要采用II类变更,B.II.d.1.e(变更批准的标准限度范围)。
Change (widening) the dissolution limits of the finished product to the limits in the Ph. Eur. monograph: Type II, B.II.d.1.e (Change outside the approved specification limits range). It is important to note that such a change should be justified by new in vivo data (see also Question 10).
变更(放宽)制剂溶出度限度和欧洲药典各论保持,需要采用II类变更,B.II.d.1.e(变更批准的标准限度范围)。重要的是要注意到,这样的变更应该通过新的体内数据来证明合理性(参见问题10)。
第二部分:EDQM的管理流程
在阅读和分析完上面EMA的问答文件,必须看看EDQM如何说。为什么呢?因为药品在欧盟注册存在特殊情况。上面提到的EMA问答只适用于制剂产品,对于原料药不是很适用。EDQM不仅发布欧洲药典,还负责维护CEP认证程序。那么,如果欧洲药典更新了版本,原料药企业如何应对呢?
2021年11月,EDQM采用新通则5.26《Implementation of pharmacopoeial procedures》,对于药典实施细节,进行厘清。
每次欧洲药典更新,EDQM官网会发布提醒,告知那些CEP持有人会受到影响。
例如,2023年7月17日,EDQM发布《Implementation of the European Pharmacopoeia Supplement 11.3 – Notification for CEP holders》。
Supplement 11.3 of the European Pharmacopoeia (Ph. Eur.) is now available. Holders of Certificates of suitability to the monographs of the Ph. Eur. (CEPs) are invited to update their applications according to the revised monographs that will be implemented on 1 January 2024, and to follow the instructions given below.
欧洲药典(Ph. Eur.)的增补版11.3现已发布。持有欧洲药典各论适应性证书(CEPs)的持有人被提醒根据将于2024年1月1日实施的修订各论更新API注册申请资料,并遵循以下给出的说明。
Thetable at the end of this announcement provides a list of substances covered by a CEP and for which a revised monograph will be implemented on1 January 2024 in Supplement 11.3of the Ph. Eur.
这个公告的末尾提供了一个表格,列出了一份由CEP认证覆盖的物质清单,这些物质将在2024年1月1日实施《欧洲药典》第11.3增补版中收载修订的药典方法。
According to Directives 2001/83/EC and 2001/82/EC, as amended, it is the responsibility of the manufacturer to comply with the current version of a Ph. Eur. monograph, and therefore to update the specification when a revised monograph is issued. In addition, the European Directorate for the Quality of Medicines and HealthCare (EDQM) ensures that CEPs refer to the most recent version of a Ph. Eur. monograph at any time.
根据2001/83/EC指令和2001/82/EC指令以及修订案,制药厂商有责任遵守欧洲药典的最新版本,并在修订的药典发布后更新质量标准。此外, EDQM确保CEP随时参考药典的最新版本。
The need to submit information to the EDQM following a revised monograph depends on the changes made to the monograph. Updates to the monographs are classified by the EDQM into two categories, labelled “Case A” and “Case B”, and this influences the information required. In the list of revised monographs below, it is indicated which classification (“Case A” or “Case B”) is applicable. In addition to this web announcement, the EDQM, as a courtesy, will contact CEP holders with details of how to proceed for the dossiers impacted by the revised monograph(s). However,it remains the responsibility of the CEP holder to comply with the requirements of the monographand if necessary to update their respective applications by the implementation date of the revised monograph at the latest, regardless of whether they have been contacted by the EDQM.
为了符合修订后药典各论,是否需要向EDQM提交信息取决于对药典所做的更改情况。EDQM将对药典的更新分为两类,分别标记为“情形A”和“情形B”,并且这种分类会影响所需提交的信息。在下面的修订药典各论列表中,会标示适用的分类(“情形A”或“情形B”)。此外,作为一种礼貌措施,EDQM将与CEP持有人联系,并提供有关受修订药典影响的申报资料如何进行修改的详细信息。然而,CEP持有人有责任遵守药典的要求,并在修订药典的实施截止日期之前更新其相应的申请,无论是否受到EDQM的联系。
Case A-The specification of the substance should be updated according to the revised monograph. Unless the CEP holder has made reference to the “current version of the monograph” (without providing details on the Ph. Eur. tests and methods in the CEP application), the updated specification should be included in the next request for revision that is submitted to the EDQM (minor, major or renewal of the certificate) and identified as such at that time (such an update will be free of charge). Where the CEP holder has made reference to the “current version of the monograph”, the revised monograph should be implemented without the need to update the specification of the substance at the next request for revision.
根据修订的药典,物质的标准应进行更新。除非CEP持有人参考了“当前版本的药典”(在CEP申请中未提供Ph.Eur.测试和方法的细节),更新后的药典标准应包含在提交给EDQM的下一个修订申请(包括小修订、大修订或证书的续订)中,并在那时进行标识(此类更新将免费)。如果CEP持有人参考了“当前版本的药典”,则无需在下一个修订申请中更新物质的标准,而应直接实施修订后的药典。
Case B-This case concerns amendments to the monograph which require the submission of data to the EDQM.
情形B-本情形涉及对药典标准的修订,要求提交数据给EDQM。
An updated dossier demonstrating that the substance complies with the requirements of the revised monograph should be provided within three months of the EDQM contacting the CEP holder. The company is asked to provide a Module 1 briefly discussing the changes made to the application. This module should also include a clarification on whether all related substances are controlled using only the method described in the revised monograph and whether the substance contains any impurities which are not described in the revised monograph (and which are found above the reporting threshold of the Ph. Eur. general monograph 2034).
EDQM联系CEP持有人后的三个月内,应提供更新的申报资料,以证明该物质符合修订药典的要求。公司被要求提供CTD模块1,简要讨论对申请所做的变更情况。此模块还应澄清是否所有有关物质仅使用修订药典中描述的方法进行控制,并且该物质是否含有任何未在修订药典中描述的杂质(且超过欧洲药典通论2034中设定的报告阈值)。
Module 3 should be updated to include, as necessary:
必要时,模块3应该被更新以包括如下信息:
a comparison of the impurity profile of the substance with the updated transparency list of the monograph (3.2.S.3.2 “Impurities”, 3.2.S.4.5 “Justification of Specifications”);
将物质的杂质档案与修订后的药典透明度列表进行比较。(涉及3.2.S.3.2杂质, 3.2.S.4.5标准论证部分)
a discussion on the suitability of the revised monograph to control any impurities which are not described in it;
讨论是否修订后的药典各论可以控制药典各论正文未涵盖的任何杂质的能力。
an updated substance specification/test methods description (3.2.S.4.1 “Specifications”, 3.2.S.4.2 “Analytical Procedures”);
更新后的物质标准/测试方法描述信息。(涉及3.2.S.4.1标准, 3.2.S.4.2分析规程部分)。
certificates of analysis of two batches with reference to the revised monograph (3.2.S.4.4 “Batch Analysis”);
根据修订后药典各论进行测试的2批次物质的COA(涉及3.2.S.4.4批分析部分)。
validation and cross-validation data when an in-house method is used as an alternative to a new test method in the monograph (3.2.S.4.3 “Validation of Analytical Procedures”).
当一种内部方法被用作药典各论的新的替代测试方法(涉及3.2.S.4.3分析方法验证),需要提供验证和交叉验证数据。
In the “Case B” scenario, CEP applications require an update and therefore any holder of a CEP for a substance in the “Case B” list below is expected to provide the requested information to the EDQM, even if no specific request for information was received (this may happen namely when information regarding a change of contact person has not been submitted to the EDQM in a timely manner).
在情形B中,CEP证书申请需要更新,因此,在下面的“情形B”列表中持有物质的CEP持有人被期望向EDQM提供所请求的信息,即使没有收到具体的信息请求(这可能发生在未及时向EDQM提交联系人变更信息的情况下)。
If the requested information has already been presented in the approved dossier, a simple letter stating this is deemed sufficient.
如果所请求的信息已经在批准的申报资料中提供过,一封简单说明函被认为是足够的。
Failure to update the CEP application and to provide data to the EDQM may challenge the validity of the concerned granted CEP, or delay the ongoing evaluation process of the concerned application.
未更新CEP申请并向EDQM提供数据可能会对已获得的CEP证书相关有效性构成挑战,或者延迟正在进行的申请的评估过程。
Upon receipt, data will be reviewed within three months and the CEP holder will be informed of the outcome of the evaluation. The assessment may also result in arevised CEPbeing granted.
收到数据后,数据将在三个月内进行审核,并将通知CEP持有者评估结果。评估可能会导致对CEP进行修订并重新授予。
This procedure is free of charge, unless the holder submits, at the same time, a request for other changes.
这个程序是免费的,除非持有人同时提交其他变更的请求。
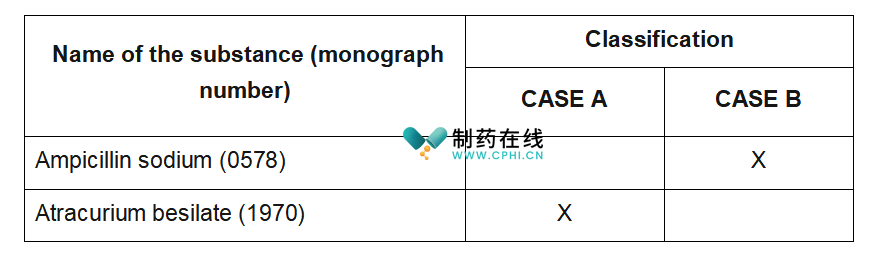
作者补充说明:上述表格是对EDQM文件中表格的简化,原表格内容很多。这里只保留2个品种,以展示药典修订后CEP持有人应该采取的行动。
Note: The Ph. Eur. monograph for Donepezil hydrochloride monohydrate (3067) is suppressed in Ph. Eur. Suppl. 11.3, as the revised monograph for Donepezil hydrochloride (2582) will cover the anhydrous and monohydrate forms of donepezil hydrochloride. EDQM will provide specific guidance to CEP holders on how this change will be managed by EDQM shortly. No action is required by CEP holders at this time.
注释:《欧洲药典》第11.3版补充版中取消了Donepezil盐酸单水合物的Ph. Eur.各论(3067),因为已修订的Donepezil盐酸盐的各论(2582)将涵盖Donepezil盐酸的无水和单水合物2种形式。EDQM将很快向CEP持有人提供关于EDQM如何管理这一变更的具体指导。CEP持有人目前无需采取任何行动。
分析:上面EDQM对CEP证书持有人发出这些涉及标准变更的要求,内在逻辑是持有人持有的证书是CEP;如果CEP证书持有人不及时更新API的标准来符合最新版欧洲药典,这个证书还叫CEP吗?
自然,如果一个API公司如果递交ASMF,而不是持有CEP证书,那么当欧洲药典更新版本时,就不一定需要采取上述变更措施。
总结
在撰写完毕上面这篇不短的文章后,笔者不得不感叹:
第一.不管是对制剂产品的标准管理,还是对持有CEP证书的API公司的管理,EMA和EDQM都提供了详尽的技术说明,可谓耗费苦心。
第二.企业的经营像呼吸一样,一刻都不能停止。EMA和EDQM深刻认识到这一点。因此尽管提醒制剂商和CEP持有人要及时更新药品标准,但是也将药品标准程序设置的比较明晰简洁,确保不会因为这些变更导致市场短缺。
第三.不仅如此,和EMA和EDQM沟通过程中,对方邮件回复速度和提供信息的详尽程度,也让申请人对于变更感受不到慌乱,因为技术支持可以很便捷获得。
作者简介:zhulikou431,高级工程师、PDA会员、ISPE会员、ECA会员、PQRI会员、资深无菌GMP专家,在无菌工艺开发和验证、药品研发和注册、CTD文件撰写和审核、法规审计、国际认证、国际注册、质量体系建设与维护领域,以及无菌检验、环境监控等领域皆具有较深造诣。近几年开始着力关注制药宏观领域趋势分析和制药企业并购项目的风险管理工作。




合作咨询
![]() 肖女士
肖女士
![]() 021-33392297
021-33392297
![]() Kelly.Xiao@imsinoexpo.com
Kelly.Xiao@imsinoexpo.com
 2006-2025 上海博华国际展览有限公司版权所有(保留一切权利)
沪ICP备05034851号-57
2006-2025 上海博华国际展览有限公司版权所有(保留一切权利)
沪ICP备05034851号-57