2024年1月8日,FDA的CDER发布了《2023年新药治疗审批报告》,从这个审批报告中我们可以了解到,2023年FDA批准了55种创新药物,这个数量远超于2022年的37种。另外,值得我们注意的是这55款获批新药中单孤儿药就高达28个,占获批药物总数的51%(详见图1)。
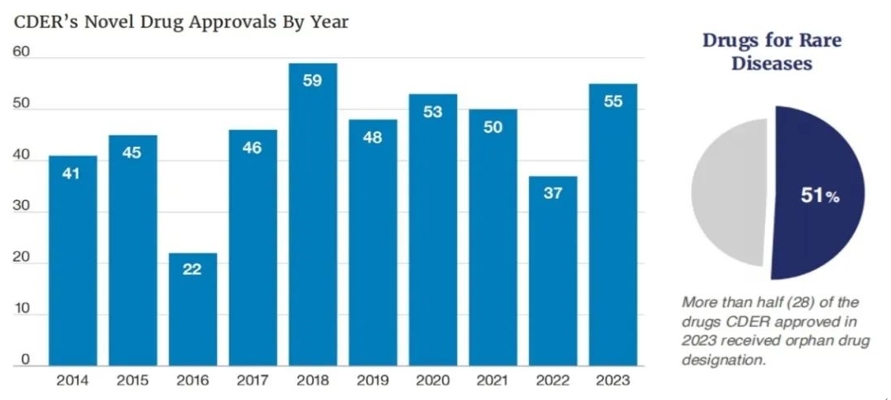
图1 CDER各年度新药批准数量
美国于1983年颁布了《孤儿药法案》,成为世界上第一个为罕见病药物立法的国家。之后为了鼓励罕见病药物的研发,美国通过了孤儿药认定(Orphan Drug Designation, ODD)计划的一系列政策。孤儿药认定(Orphan Drug Designation, ODD)就是FDA 孤儿药产品开发办公室(Office of Orphan Products Development, OOPD) 对符合条件的用于预防、治疗及诊断罕见病的药物(包括生物制品)授予的一种资格认定。
近年来,国内多家生物医药公司开发的创新药在美国获得孤儿药资格认定,本文我们就盘点一下2023年中国制药企业获得FDA孤儿药资格认定的创新药。
根据FDA的孤儿药认定数据库资料初步统计,2023年获得FDA授予的孤儿药认定的创新药共有376种,其中中国的创新药占59种(按适应症计),约占15%(详见表1)。
国内制药企业获得FDA孤儿药资格认定的这些产品,从其适应症领域来看,多集中在肿瘤领域,占比高达63%(37/59),数量最多的5个癌种依次是胰腺癌(9项)、胃癌(5项)、恶性胶质瘤(4项)、小细胞肺癌(3项)、胆道癌(2项)。除此之外,肌萎缩侧索硬化症和亨廷顿舞蹈症关注度也较高。
另外,这些产品涵盖了CAT-T疗法、抗体偶联药物(ADC)、双特异性抗体、基因疗法等创新疗法。
值得注意的是,有多家公司的多个产品同时被授予孤儿药资格认定。例如博安生物自主开发的两款靶向Claudin18.2的在研产品--创新抗体BA1105、创新抗体偶联药物BA1301获得FDA授予的治疗胰腺癌适应症的孤儿药资格认定;Suzhou Immunofoco Biotechnology Co., Ltd(易慕峰)是一家致力于突破实体瘤治疗,给患者带来长期生存获益的免疫细胞治疗药物开发企业,其自主研发的靶向EpCAM的自体CAR-T细胞注射液产品获得FDA授予的治疗胃癌适应症的孤儿药资格认定,时隔不久该产品又被FDA授予孤儿药资格认定,用于治疗胰腺癌患者;德琪(杭州)生物自主研发和开发的Claudin 18.2抗体偶联药物(ADC)ATG-022获FDA先后授予的两项孤儿药资格认定,分别用于治疗胃癌及胰腺癌。
表1 2023年获得FDA授予孤儿药资格认定的中国药企及产品
|
NO. |
药品名称 |
授予时间 |
适应症 |
MAH |
|
1 |
4-(3-(4-(1H-imidazo[4,5-b]pyridin-5-yl)piperazine-1-carbonyl)-4-fluorobenzyl)phthalazin-1(2H)-one hydrochloride dihydrate |
01/09/2023 |
小细胞肺癌(SCLC) |
Shanghai Yidian Pharmaceutical Technology Development Co., Ltd. 上海壹典医药科技开发
|
|
2 |
recombinant adeno-associated virus serotype 8 vector encoding human phenylalanine hydroxylase (PAH) |
01/09/2023 |
苯丙酮尿症(PKU) |
NGGT (Suzhou) Biotechnology Co., Ltd. |
|
3 |
N-(6-(2,6-dichloro-3,5-dimethoxyphenyl)-1H-pyrazolo[3,4-b]pyridin-3-yl)-4-(3,3-dimethylpiperazin-1-yl)benzamide hydrochloride |
01/12/2023 |
胃癌和胃食管交界癌 |
3D Medicines (Beijing) Co., Ltd |
|
4 |
(S)-N-(5-(1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethyl)-4,6-dioxo-5,6-dihydro-4H-thieno[3,4-c]pyrrol-1-yl)acetamide |
01/18/2023 |
白塞病 |
Ganzhou Hemay Pharmaceutical Co., Ltd.合美医药 |
|
5 |
5¿-(4-fluorophenyl)-3¿-isopropyl-N-(4-(4-methylpiperazin-1-yl) phenyl)-1H,3¿H-[2,4¿-biimidazole]-4-carboxamide acetate |
02/01/2023 |
特发性肺纤维化 |
InSilico Medicine Hong Kong Limited英矽智能 |
|
6 |
(-)-B-Elemene( KH617) |
02/14/2023 |
多型胶质母细胞瘤 |
Sichuan Honghe Biotechnology Co., Ltd.四川弘合生物科技 |
|
7 |
a humanized, immunoglobulin G subtype 4 anti-Amphiregulin monoclonal antibody |
02/16/2023 |
特发性肺纤维化 |
Pulmongene (Hong Kong) Co., Limited普沐(香港)生物科技有限公司 |
|
8 |
allogeneic T cells genetically modified to express anti-B7-H3 chimeric antigen receptor (CAR) |
02/16/2023 |
恶性胶质瘤 |
T-MAXIMUM Pharmaceutical (Suzhou) Co., Ltd. |
|
9 |
Mefuparib |
02/28/2023 |
胆道癌 |
Convalife (Shanghai) Co., Ltd.甫康(上海)健康科技 |
|
10 |
siRNA oligonucleotide designed to target and knockdown the expression of SOD1 |
03/01/2023 |
肌萎缩侧索硬化症(ALS) |
Ractigen Therapeutics中美瑞康 |
|
11 |
Autologous CD4+/CD8+ T cells transduced with lentiviral vector directed against MAGE-A4 antigen |
03/23/2023 |
IIB至IV期黑色素瘤 |
TCRx Therapeutics Ltd.科士华生物 |
|
12 |
Humanized, Low-fucose Anti-Claudin 18.2 IgG1 Antibody |
03/23/2023 |
胰腺癌 |
Suzhou Transcenta Therapeutics Co., Ltd.创胜集团 |
|
13 |
3-(5-cyano-4-(cyclopropylamino)pyridin-2-yl)-1-(6-formyl-5-((4-methyl-2-oxopiperazin-1-yl)methyl)pyridin-2-yl)-1-methylurea |
03/27/2023 |
软组织肉瘤 |
Wuxi Abbisko Biomedical Technology Co., Ltd.和誉生物医药 |
|
14 |
a multiple substituted pyrimidoheterocycle small molecule inhibitor that covalently binds to the Switch II pocket of KRAS p.G12C and locks the oncogenic protein in its GDP-bound inactive form |
03/27/2023 |
携带KRAS p.G12C突变蛋白的晚期癌症 |
D3 Bio (Wuxi) Co., Ltd.德昇济 |
|
15 |
A plasmid encoding a rabies virus glycoprotein tag & lysosome-associated membrane glycoprotein 2 fusion protein gene and a mutant huntingtin small interfering RNA |
04/10/2023 |
亨廷顿舞蹈症 |
ExoRNA Bioscience Nanjing Co. Ltd. |
|
16 |
(2-(6-(2-(4-cyclopropylpyrimidin-5-yl)-4-fluorophenoxy)-1,2,4-triazin-5-yl)-2,7-diazaspiro[3.5]nonan-7-yl)((1S,3aR,6aS)-octahydrocyclopenta[c]pyrrol-1-yl)methanone |
04/17/2023 |
急性髓细胞白血病 |
BioNova Pharmaceuticals (Shanghai) Limited烨辉医药 |
|
17 |
Edralbrutinib |
04/24/2023 |
视神经脊髓炎谱系障碍 |
Reistone Biopharma Company Limited瑞石生物医药 |
|
18 |
allogeneic umbilical cord mesenchymal stem cells |
05/03/2023 |
眼部移植物抗宿主病 |
Guangdong ProCapZoom Biosciences Co., Ltd.广东普罗凯融生物医药 |
|
19 |
Autologous Chimeric Antigen Receptor (CAR) Modified Recombinant T Cells Targeting EGFRvIII |
05/09/2023 |
恶性胶质瘤 |
Beijing DCTY® Biotech Co., Ltd.北京鼎成肽源生物技术 |
|
20 |
N-[3-fluoro-4-[[1-methyl-6-(1H-pyrazol-4-yl)-1H-indazol-5-yl]oxy]phenyl]-1-(4-fluorophenyl)-1,2-dihydro-6-(1-methylethoxy)-2-oxo-3-pyridinecarboxamide(多靶点激酶抑制剂TSN084) |
05/16/2023 |
用于治疗急性髓系白血病 |
Tyligand Bioscience (Shanghai) Limited泰励生物 |
|
21 |
Antibody drug conjugate targeting Claudin 18.2 (CLDN 18.2) consisting of a humanized monoclonal antibody against CLDN 18.2 and a conjugated linker-payload VC-MMAE (maleimidocaproyl-valine-citrulline-p-aminobenzylalcohol-Monomethyl Auristatin E) |
05/16/2023 |
胰腺癌 |
Antengene (Hangzhou) Biologics Co., Ltd.德琪(杭州)生物 |
|
22 |
autologous CD3+ T cell product transduced with transgene constructs expressing anti-human mesothelin (MSLN) CAR and self-secreting anti-human PD-1 nanobody |
05/16/2023 |
间皮瘤 |
Shanghai Cell Therapy Group Pharmaceutical Technology Co., Ltd上海细胞治疗集团 |
|
23 |
antibody drug conjugate targeting Claudin 18.2 (CLDN 18.2) consisting of a humanized monoclonal antibody against CLDN 18.2 and a conjugated linker-payload VC-MMAE (maleimidocaproyl-valine-citrulline-p-aminobenzylalcohol-Monomethyl Auristatin E) |
05/22/2023 |
胃癌 |
Antengene (Hangzhou) Biologics Co., Ltd德琪(杭州)生物 |
|
24 |
Recombinant humanized anti-MSLN monoclonal antibody- monomethyl auristatin E conjugate |
05/22/2023 |
胰腺癌 |
RemeGen Co., Ltd荣昌生物 |
|
25 |
chimeric antigen receptor (CAR) modified recombinant T cells targeting B7-H3 |
06/21/2023 |
恶性胶质瘤 |
Fuzhou Tcelltech Biological Science and Technology Inc. |
|
26 |
Pegylated Iron Oxide Nanoparticles |
06/26/2023 |
肝细胞癌的治疗的诊断 |
MegaPro Biomedical Company, Ltd.巨生生医 |
|
27 |
human umbilical cord-derived mesenchymal stem cells |
08/09/2023 |
特发性肺纤维化 |
Wuhan Optics Valley Vcanbiopharma Co., Ltd. |
|
28 |
Autologous synthetic NKG2D receptor armored CLDN18.2 specific CAR-T cell injection |
08/15/2023 |
胃癌 |
Suzhou Immunofoco Biotechnology Co., Ltd易慕峰 |
|
29 |
Autologous EpCAM specific CAR-T cell injection |
08/16/2023 |
胃癌 |
Suzhou Immunofoco Biotechnology Co., Ltd易慕峰 |
|
30 |
a genetically modified Salmonella comprising of methioninase |
08/28/2023 |
小细胞肺癌 |
Guangzhou Sinogen Pharmaceutical Co., Ltd.广州华津医药 |
|
31 |
Autologous synthetic NKG2D receptor armored CLDN18.2 specific CAR-T cell injection |
08/28/2023 |
胰腺癌 |
Suzhou Immunofoco Biotechnology Co., Ltd易慕峰 |
|
32 |
autologous chimeric antigen receptor (CAR) T cells targeting CD7 |
08/31/2023 |
急性淋巴细胞白血病和淋巴细胞淋巴瘤 |
Hebei Senlang Biotechnology Co. Ltd.河北森朗生物 |
|
33 |
N( 4{[ 5fluoro7( 2methoxyethoxy) 4quinazolinyl] amino}phenyl)2( 4isopropyl1H1,2,3triazol1yl) acetamide 4-methylbenzenesulfonate (1:1) |
09/13/2023 |
胃肠道间质瘤(GIST) |
Ningbo NewBay Technology Development Co., Ltd.宁波新湾医药 |
|
34 |
(R)-3-(5,5-dimethyl-4,5-dihydro-1,2,4-oxadiazol-3-yl)-N-(1-(2,3,5-trifluorophenyl) ethyl) pyrazolo[1,5-a] pyrimidin-5-amine hydrate |
09/19/2023 |
ALK阳性、ROS1阳性、NTRK融合阳性或LTK阳性非小细胞肺癌 |
TYK Medicines, Inc 同源康 |
|
35 |
Folate-TRPV6-peptide exatecan drug conjugate (CBP-1019) |
09/19/2023 |
胰腺癌 |
Coherent Biophanna (Hefei) Co., Ltd.同宜医药 |
|
36 |
Human Retinal Pigment Epithelial Cell Injection |
09/19/2023 |
色素性视网膜炎 |
Eyecure Therapeutics Inc.江苏艾尔康生物医药科技 |
|
37 |
A Lipid Nanoparticle Suspension of Akt-1 Antisense Oligonucleotide |
09/20/2023 |
肝细胞癌 |
Zhejiang Haichang Biotech Co., Ltd.浙江海昶生物医药技术有限公司 |
|
38 |
((2S,5S)-5-(4-amino-5-(4-(2,3-difluorophenoxy)phenyl)imidazo[5,1-f][1,2,4]triazin-7-yl)tetrahydro-2H-pyran-2-yl)methanol |
10/02/2023 |
用于治疗中枢神经系统淋巴瘤 |
Dizal (Jiangsu) Pharmaceutical Co., Ltd.迪哲(江苏)医药 |
|
39 |
recombinant adeno-associated virus vector serotype 8 carrying the coding sequence of human GBA1 gene |
10/05/2023 |
戈谢病 |
Lingyi Biotech Co. Ltd凌意(杭州)生物科技 |
|
40 |
Virus-like particle containing Cas9/gRNA ribonucleoprotein targeting the human HTT gene |
10/05/2023 |
亨廷顿舞蹈症 |
Shanghai BDgene Co., Ltd.上海本岛基因 |
|
41 |
adeno-associated virus (AAV) vector-based gene therapy delivering a therapeutic transgene of RJK002 |
10/16/2023 |
肌萎缩侧索硬化症 |
Rejukon Biopharm Inc.瑞吉康生物医药有限公司 |
|
42 |
recombinant humanized anti-interleukin 36R monoclonal antibody |
10/16/2023 |
泛发性脓疱型银屑病 |
Shanghai Huaota Biopharmaceutical Co., Ltd.上海华奥泰生物药业 |
|
43 |
N-(2-(4-Fluorophenyl)-5,7-dimethyl-1,2,3,4-tetrahydro isoquinolin-6-yl)-2-(1-methylcyclopropyl) acetamide |
10/18/2023 |
肌萎缩侧索硬化症 |
Shanghai Zhimeng Biopharma, Inc上海挚盟医药科技 |
|
44 |
Recombinant human signal regulating protein alpha (SIRPalpha) fragment crystallizable (Fc)-fusion protein |
11/07/2023 |
慢性粒单核细胞白血病(CMML) |
ImmuneOnco Biopharmaceuticals (Shanghai) Inc.宜明昂科 |
|
45 |
Recombinant human IgG1 anti-B7H3 monoclonal antibody conjugated to topoisomerase I inhibitor YL0010014 |
11/16/2023 |
食管癌 |
MediLink Therapeutics苏州宜联生物医药 |
|
46 |
Allogenic spinal motor neuron precursor cell |
11/22/2023 |
肌萎缩侧索硬化症(ALS) |
XellSmart Bio-Pharmaceutical (Suzhou) Co., Ltd士泽生物医药(苏州) |
|
47 |
Epirubicin |
11/28/2023 |
恶性胶质瘤 |
Beijing Inno Medicine Co., Ltd.茵诺医药 |
|
48 |
Chaenomelis Fructus Extract |
11/29/2023 |
BAG3相关肌原纤维肌病 |
Centre for Chinese Herbal Medicine Drug Development Limited中药创新研发中心 |
|
49 |
a bispecific antibody composed of a Light Chain and a Heavy Chain pair targeting HER2, and a Light Chain and a Heavy Chain pair targeting CD47 |
12/07/2023 |
胃癌症患者的治疗,包括胃食管交界处癌症 |
D3 Bio (Wuxi) Co., Ltd.德昇济 |
|
50 |
Tetrahydro-2H-pyran-4-yl 7-(2-(cyclopropanecarboxamido)- imidazo[1,2-a]pyridin-6-yl)-2,3-dihydro-1H-pyrido[2,3-b][1,4]oxazine-1-carboxylate hydrochloride |
12/11/2023 |
急性移植物抗宿主病 |
Accro Bioscience (Suzhou) Limited爱科诺生物医药(苏州)有限公司 |
|
51 |
(-)-Huperzine A石杉碱甲 |
12/12/2023 |
重症肌无力 |
Wanbangde Pharmaceutical Group Co, Ltd.万邦德制药 |
|
52 |
recombinant humanized anti-human tissue factor monoclonal antibody conjugated with a cytotoxic small molecule MMAE |
12/12/2023 |
胰腺癌 |
Shanghai Miracogen Inc.上海美雅珂生物 |
|
53 |
trastuzumab-rezetecan |
12/14/2023 |
胆道癌 |
Jiangsu Hengrui Pharmaceuticals Co., Ltd. 恒瑞医药 |
|
54 |
recombinant fusion protein composed of a recombinant human arylsulfatase A (rhARSA) fused with a variable domain of the heavy chain of heavy chain-only antibody fragment targeting human transferrin |
12/15/2023 |
异染性脑白质营养不良 |
Linno Pharmaceuticals, Inc.领诺医药 |
|
55 |
an IgG1(Immunoglobulin G1) subclass antibody targeting CCR8(C-C Motif Chemokine Receptor 8) |
12/18/2023 |
胰腺癌 |
LaNova Medicines Limited礼新医药 |
|
56 |
Adeno-associated viruses 8 (AAV8)-based gene therapy vector that expresses B domain-deleted human factor VIII under the control of human liver-specific promoters |
12/19/2023 |
血友病A |
Gritgen Therapeutics Co., Ltd.华毅乐健 |
|
57 |
anti-Claudin18.2 monoclonal antibody conjugated with Duostatin-5 payload |
12/21/2023 |
胰腺癌 |
Shandong Boan Biotechnology Co., Ltd.博安生物 |
|
58 |
Human monoclonal IgG1 antibody against the isoform 2 of human Claudin 18 protein (Claudin 18.2) |
12/21/2023 |
胰腺癌 |
Shandong Boan Biotechnology Co., Ltd.博安生物 |
|
59 |
Dual recombinant adeno-associated viral vectors encoding the human otoferlin gene |
12/21/2023 |
耳啡肽介导的听力损失 |
Shanghai Refreshgene Therapeutics Co., Ltd.上海鼎新基因科技 |
分析
从上面这些数据看,国内创新药企业对美国孤儿药的政策很熟悉,并且充分运用。对于研发成本高启的美国医药市场,可以通过孤儿药资格认定这条途径,开启合适的临床试验并控制研发费用,对于创新药公司是一条不错的开辟途径。
参考资料:
1-FDA官网
2-FDA《2023年新药治疗审批报告》
作者简介:zhulikou431,高级工程师、PDA会员、ISPE会员、ECA会员、PQRI会员、资深无菌GMP专家,在无菌工艺开发和验证、药品研发和注册、CTD文件撰写和审核、法规审计、国际认证、国际注册、质量体系建设与维护领域,以及无菌检验、环境监控等领域皆具有较深造诣。近几年开始着力关注制药宏观领域趋势分析和制药企业并购项目的风险管理工作。




合作咨询
![]() 肖女士
肖女士
![]() 021-33392297
021-33392297
![]() Kelly.Xiao@imsinoexpo.com
Kelly.Xiao@imsinoexpo.com
 2006-2025 上海博华国际展览有限公司版权所有(保留一切权利)
沪ICP备05034851号-57
2006-2025 上海博华国际展览有限公司版权所有(保留一切权利)
沪ICP备05034851号-57